June 2021 Newsletter:
Polyganics’ Sealing Device is indicated for use to reduce post-operative leakage of fluids from the site of surgery into the abdominal cavity after Hepato-Pancreato-Biliary (HPB) surgery, and as an adjunctive hemostatic device to control minimal to moderate bleeding.

Welcome to this issue of Polyganics’ SHIELDS* newsletter
Welcome to a newest issue of our SHIELDS* newsletter, which is intended to keep you, our clinical partners, up to date with the latest on the clinical trial.
With more than 65 patients enrolled already, we are in a good position to complete enrollment of all 80 patients by the end of June 2021. This has only been possible thanks to your considerable support. Thank you all for your significant efforts to drive progress despite the limitations to OR capacity and IC space caused by COVID-19.
In this issue, look out for:
- An update on the active clinical sites and patients enrollment
- Our approach to finalizing enrollment of all 80 patients by end of June 2021
- Our approach to data collection and completion
- Your invite to our Investigator Meeting – June 24, 2021 (17-19 CET)- RSVP before June 17
- Next steps: SHIELDS II: a randomized controlled study for approval in the US
- Your invite to join us at the E-AHPBA Bilbao online event
Thank you for reading our newsletter. If you have comments, questions, or we can help further in any way to support your participation in the SHIELDS clinical study, please get in touch.
*SHIELDS: Prospective, multicenter study to evaluate the Safety and performance of a syntHetic tissue sealant in reducing fluId lEakage following elective hepatobiLiary anD pancreatic Surgery
An update on active clinical sites and patients enrollment
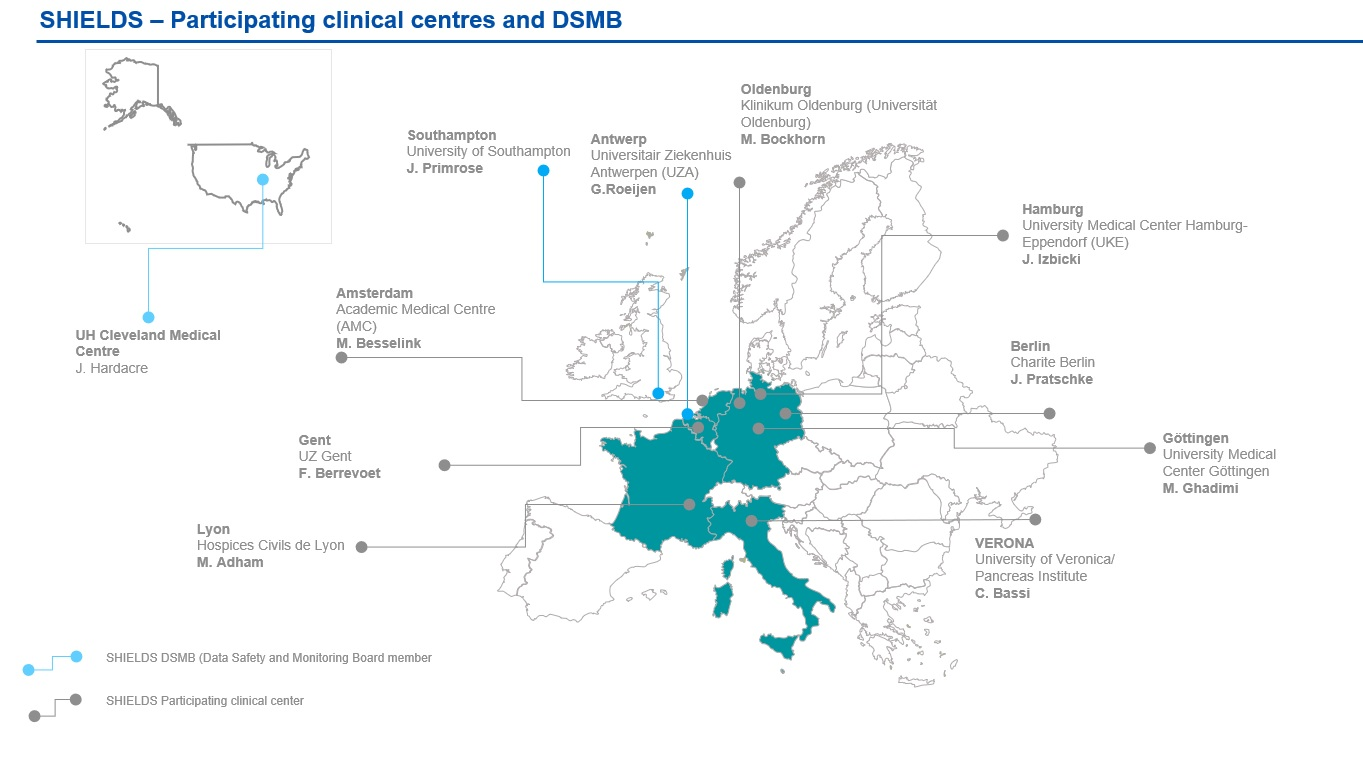
As a multicenter, prospective study, SHIELDS involves eight European sites, across Belgium, France, Germany, Italy and The Netherlands. All eight of these sites are actively enrolling patients. Figure 1 shows an overview of the participating clinical centres and DSMB members.
Despite COVID-19 restrictions somewhat impeding study enrollment, all the sites are making considerable effort and have succeeded in enrolling patients. Our earliest sites in Hamburg, Oldenburg and Verona continue to enroll patients at a steady rate, while the newly-activated sites in Amsterdam, Berlin, Lyon, Gent and Göttingen have managed to enroll a large number of liver patients in a very short time span. In total, 66 patients have been enrolled to date (38 liver and 28 pancreas) across the participating centres. Enrollment distribution is as shown in Table 1. Please note that the site activation dates should be taken into consideration.
| Active months* | Pancreas patients enrolled | Liver patients enrolled | Total patients enrolled | |
| Hamburg | 7 | 4 | 12 | 16 |
| Oldenburg | 8 | 6 | 5 | 11 |
| Verona | 5 | 11 | – | 11 |
| Berlin | 2 | 1 | 3 | 4 |
| Göttingen | 3 | 1 | 8 | 9 |
| Gent | 3 | 2 | 5 | 7 |
| Amsterdam | 3 | 1 | 4 | 5 |
| Lyon | 1 | 2 | 1 | 3 |
Table 1: Enrollment per site
* Active enrollment period starting at first patient enrolled; excluding safety stop period Germany
Finalizing enrollment by end of June 2021
Based on recent communication with the participating clinical centres regarding enrollment rates, we anticipate that the complete study population (N = 80; of which 40 liver and 40 pancreas patients) will be finalized by the end of June 2021.
The liver cohort is already nearing completion. However, active recruitment will be extremely important to enable us to achieve this goal for pancreas patients. In all of this, the quality of the trial and its outcomes is imperative. If you are in doubt as to whether a patient is a good candidate for the study, please contact Polyganics Clinical Research Manager (Hilde Geraedts) and we will help to assess the eligibility of your potential pancreas patient.
As we approach the end of enrollment in the trial, it is of utmost importance that you, our clinical partners, proactively share with us your enrollment plans each week. We must ensure that the trial is concluded once we meet our goal of 40 patients in both the liver and pancreas groups. No patients can be included above this intended patient population, as dictated by the protocol. We will keep you up to date with the overall enrollment status and will reach out to individual sites as well with regards to progress.
Timely data collection and completion
Based on enrollment being completed by the end of June 2021, we anticipate data collection for the 30-day follow-up primary outcome to be completed by the end of July. Timely entry of the data for the 30-day and 90-day follow-up visits is essential to facilitate reporting and preparation.
Monitoring visits tailored to each site’s current regulations are being planned and performed at all sites. This is pivotal for study completion and part of working towards data finalization, ensuring data quality and patient safety.
We understand and apologize for the inconvenience the monitoring may cause due to practical challenges in current times. We thank you for the effort you are all putting in to achieve adequate monitoring, which will enable timely data cleaning and finalization for the primary endpoint at 30-days follow-up, as well as the official interim study report at 90-days.
Investigator Meeting
After a successful first Investigator Meeting (IM) on March 3, we invite all study investigators to join our second Online IM on June 24, 2021 – 5-7pm CET.
At the meeting, up-to-date results will be discussed in detail, and practical tips and any relevant updates to study procedures will be shared, which can help to enhance study performance. In addition, several investigators will present their own case studies. Finally, we will also discuss our upcoming SHIELDS II trial, which we aim to start by the end of this year (see section: Upcoming SHIELDS II study).
The meeting agenda is as follows:
- Introduction
- Protocol – short recap (including deviations and modifications for MDR compliance)
- Status trial (enrollment and planning)
- Overview latest results (interim update patient outcomes)
- Special cases highlighted
- SHIELDS-II set-up and preparations
- Planning
- Closure and other items
Presenters include, among others, Prof. Dr. Jakob Izbicki FACS FRCS ed. hon. FEBS (Coordinating study PI) and Prof. Dr. Max Bockhorn (PI Oldenburg).
Please let us know before June 17 if you will be able to join this meeting by sending an email to clinical@polyganics.com. Any questions in advance can also be submitted using this e-mail address.
We look forward to discussing the study progress and case studies with you!
Upcoming SHIELDS II study
Based on the results of our European SHIELDS study, we will submit our Liver and Pancreas Sealing Patch for CE Approval. In addition, we are preparing a second clinical trial, SHIELDS II, a randomized controlled study that will enable us to obtain approval in the US. At the upcoming Investigator Meeting we will share our thoughts on the protocol for this study. We look forward to receiving your input during that meeting.
E-AHPBA Bilbao online event
In the previous edition of this newsletter, we announced our strategic partnership with the European-African Hepato-Pancreato-Biliary Association (E-AHPBA). Recently, we have received the good news that the data collected for the first 10 of our study patients, as part of the safety cohort in Germany, have been accepted for poster presentation at the E-AHPBA Bilbao congress, September 15-17, 2021.
Polyganics intends to sponsor this online event and we warmly invite you to attend. Please let us know before July 1, 2021 whether you are able to participate. If you are unable to attend the meeting, we can provide the poster on the safety data to you by e-mail.