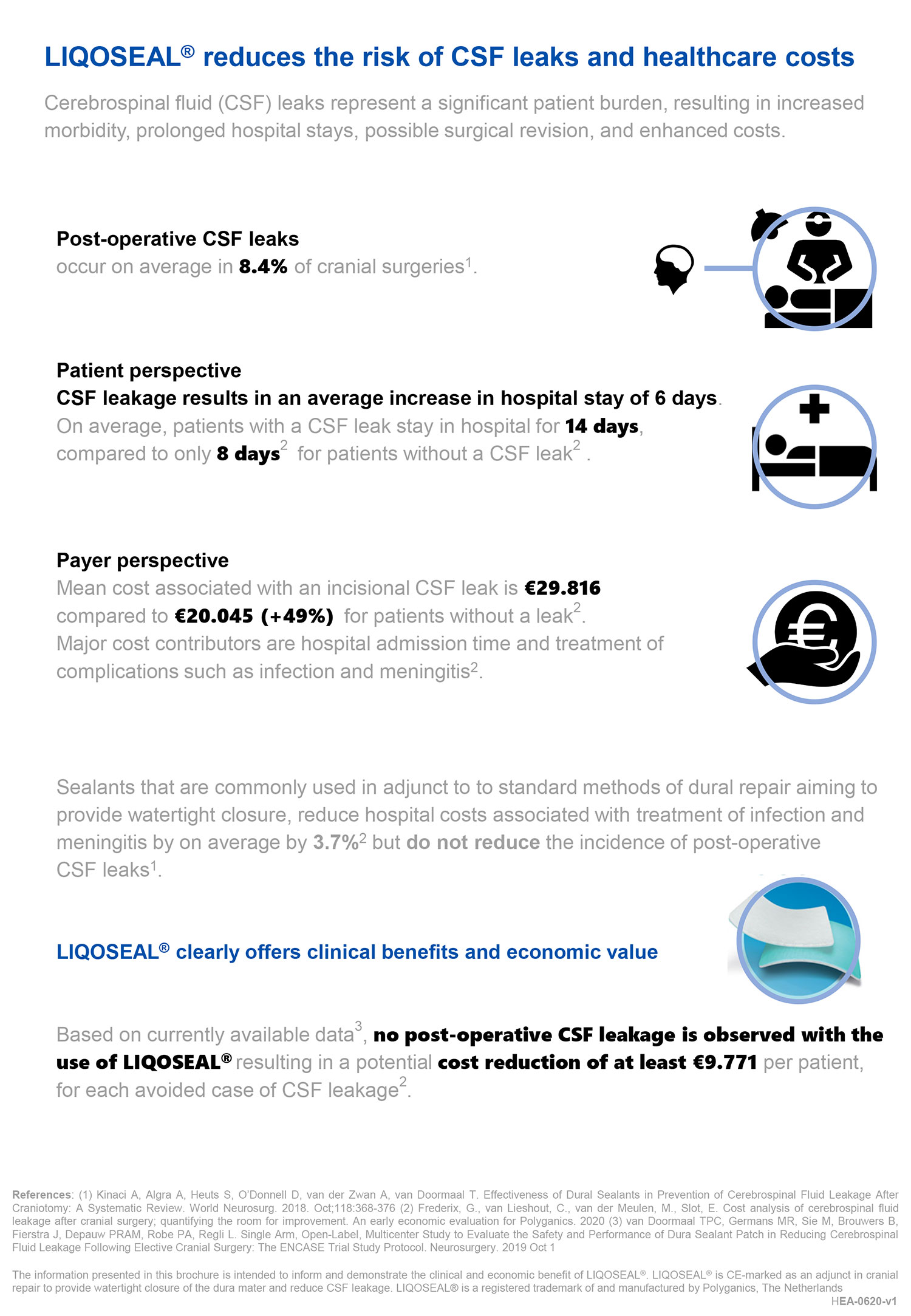
LIQOSEAL®, the first product in our Neurosurgery portfolio, is indicated for use as an adjunct to standard methods of cranial dural repair to provide watertight closure of the dura mater and reduce cerebrospinal fluid (CSF) leakage.
Update on our ENCASE I study
We are pleased to report the submission of 12-month data for ENCASE I. ENCASE I, the first-in-human trial of LIQOSEAL® is a single arm, open-label study evaluating the safety and performance of LIQOSEAL® in reducing CSF leakage following elective cranial surgery. 40 adult patients have been recruited at three sites across the Netherlands and Switzerland, with follow-up due to span a period of 12 months. The protocol of ENCASE I has been published in Neurosurgery.
As soon as 12-month data are accepted for publication, we are happy to share these results with you. 12-month data of ENCASE I will also provide a more robust estimate of the cost-saving potential of LIQOSEAL® in clinical practice.
An invitation to join us at EANS virtual congress
Polyganics is sponsoring the EANS Virtual congress (October 19-21). To introduce LIQOSEAL® to registered surgeons, Dr van Doormaal, Principal Investigator of the ENCASE I study, will share outcomes and his recommendations in an online session:
‘Single-arm, open-label, multicenter study to evaluate the safety and performance of LIQOSEAL® in reducing cerebrospinal fluid leakage following elective cranial surgery: The ENCASE Trial’
We encourage you to attend the session and to invite your customers to do the same. This is a great opportunity to connect with users and foster awareness and enthusiasm for LIQOSEAL®.
Programme at a Glance
Upcoming webinar: please register before Oct 12th
Polyganics is hosting a webinar on 15 October at 18.00 CEST:
Reducing CSF Leakage after Cranial Surgery
In this 1-hour webinar we will share results from our preclinical and clinical studies evaluating the performance of LIQOSEAL® in reducing CSF leakage. We will guide you through our ENCASE clinical trials, as well as the first clinical experiences of using LIQOSEAL® and recent investigational work.
Presenting on the webinar are:
- Andrew Carlson, MD, PhD – Neurosurgeon at University of New Mexico – Albuquerque – USA and Principal Investigator of ENCASE II
- Tristan van Doormaal, MD, PhD – Neurosurgeon at the University Hospital Zurich (Switzerland) and University Medical Center (UMC) in Utrecht (The Netherlands) and Principal Investigator of ENCASE I
- Dr Menno Germans, MD, PhD – Neurosurgeon at the University Hospital Zurich (Switzerland)
- Dr Emma Slot, MD – PhD Student Neurosurgery at University Medical Center (UMC) in Utrecht
We are extending registration to 12 October so that you still have time to invite your customers to join us. Email neurosurgery@polyganics.com to register.
Update on our post-market study, ENCASE II
To extend LIQOSEAL’s reach into the US and strengthen its position in Europe, we are preparing for the start of ENCASE II.
This randomized controlled trial will allow us to submit the device for pre-market approval to the US FDA, and will serve as a post-market clinical follow-up study in Europe, to further establish the performance of LIQOSEAL® in reducing CSF leakage.
Several renowned neurosurgery centers have already expressed interest in participating in the study, both in the US and Europe.
Reminder for our online training program
Finally, a reminder to try out and continue sharing our online training program.
Over the past few months, we have seen good uptake of the training, which aims to instruct surgeons on how to use LIQOSEAL® for dural repair.
We have developed this content both to support surgeons during these challenging times when we are unable to visit the hospital or attend the OR, and to assist you in conveying the ease-of-use and overall value of LIQOSEAL® to your customers.
To ensure everyone’s privacy, the training module is protected by a secure login. Please contact us if you need to be resent your password.