Polyganics’ Sealing Device is indicated for use to reduce post-operative leakage of fluids from the site of surgery into the abdominal cavity after Hepato-Pancreato-Biliary (HPB) surgery, and as an adjunctive hemostatic device to control minimal to moderate bleeding.

Welcome to this issue of Polyganics’ SHIELDS* newsletter
Welcome to the second issue of our SHIELDS newsletter, which is intended to keep you, our clinical partners, up to date with the latest on the study.
With more than 20 patients now enrolled, the SHIELDS study is well underway to complete enrollment of all 80 patients by April 2021. We thank you for all your significant efforts so far, which are helping to drive progress despite the ongoing global struggle against COVID-19. Please do not hesitate to contact us if any issues arise related to patient enrollment over the next couple of months.
In our previous newsletter, we provided you with an update on the start-up of clinical centres, including regulatory processes, and announced our upcoming investigator meeting. In this issue please find:
- An update on active clinical sites, patient enrollment and regulatory processes
- Your invite to our Investigator Meeting – March 3, 2021 (6-8 PM, CET) – RVSP before Feb, 26
- Announcement partnership E-AHPBA
- A reminder on how to access our online instructional videos
Thank you for reading our newsletter. If you have comments, questions, or we can help further in any way to support your participation in the SHIELDS clinical study, please get in touch.
*SHIELDS: Prospective, multicenter study to evaluate the Safety and performance of a syntHetic tissue sealant in reducing fluId lEakage following elective hepatobiLiary anD pancreatic Surgery
Update on active clinical sites, patient enrollment and regulatory processes
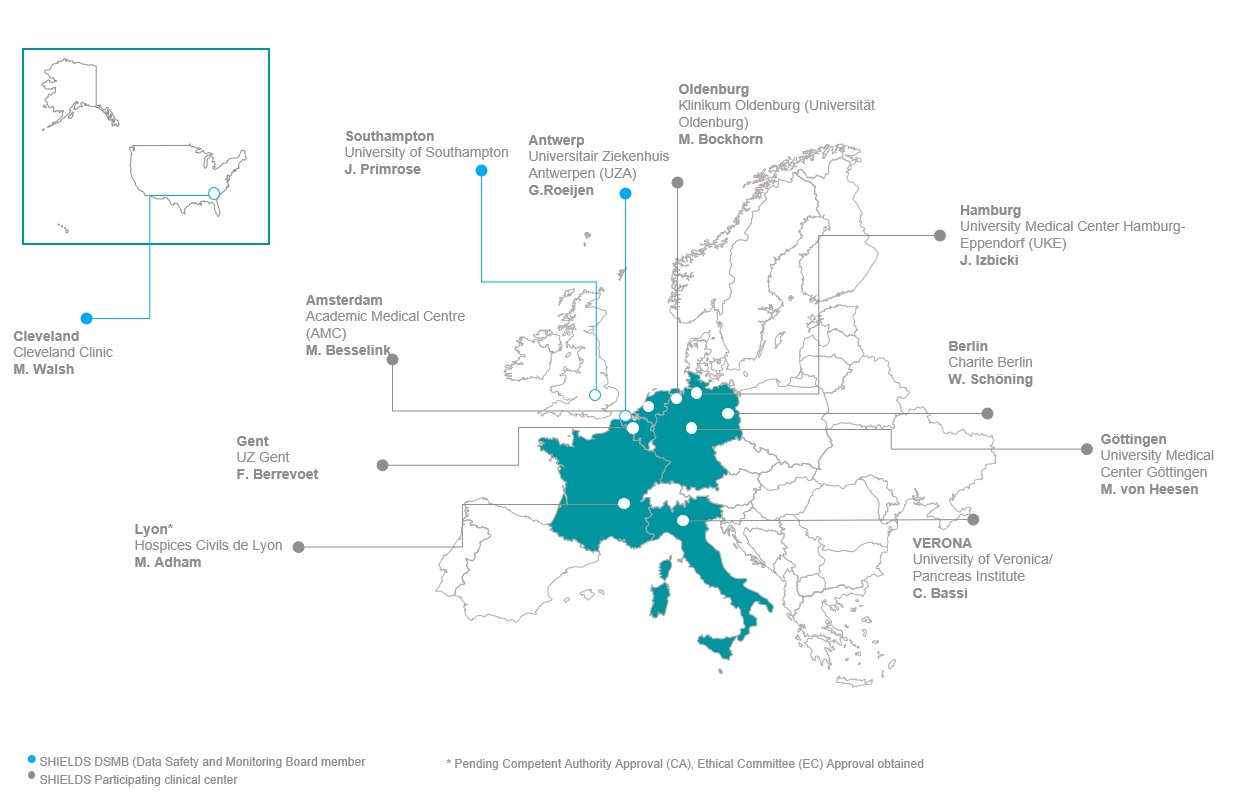
As a multicenter, prospective study, SHIELDS involves eight European sites, spread across Belgium, France, Germany, Italy and The Netherlands. Currently, 7 sites have been activated ready to enroll patients. In Figure 1, an overview of SHIELDS’ participating clinical centres and DSMB members can be found.
In our previous newsletter, we announced that enrollment in Germany would continue after the National Competent Authority (BfArM) has positively evaluated the seven-day follow-up report from the fist 10 patients. In the meantime, we received approval from BfArM to continue and 16 patients have been enrolled in Germany to date (7 in Oldenburg, 7 in Hamburg, 2 in Gottingen). Unfortunately, still restricted by strict COVID-19 regulations, no patients have yet been enrolled for the study in Berlin.
In total, 23 patients have been enrolled to date (11 liver and 12 pancreas). In addition to the 16 patients enrolled in Germany, 5 have been enrolled in Italy, 1 in Gent and 1 in Amsterdam. Our French site (Lyon) is under review by the relevant authorities and final approval is anticipated shortly
Based on recent communications with on-site teams regarding enrolment rates, we anticipate that the complete study population (N = 80; of which 40 liver and 40 pancreas patients) should be finalized by the end of April 2021.
Investigator meeting
We invite you to join the 1st Online Investigator Meeting for the study investigators, which has been planned for March 3, 2021 – 6 to 8 PM CET. During this meeting, the available results at that time will be discussed in detail and practical tips for the study performance can be shared. Relevant updates to the study procedures will be presented. In addition, several investigators will present some of their study cases in detail. The meeting agenda is as follows:
- Introduction
- Device updates (shelf life extension, storage and shipment conditions)
- Protocol: short recap and goals trial (including deviations and modifications for France and the Netherlands)
- Status trial (enrolment and planning)
- Overview latest results (interim update patient outcomes)
- Special cases highlighted
- Planning process
- Any other items
- Closing
Presentors are, among others Jakob Izbicki (JI, Coordinating study PI), Asmus Heumann (AH, PI Hamburg) and Max Bockhorn (MB, PI Oldenburg)
Please let us know before Feb 26 if you will be able to join this meeting, by clicking one of the options below or by sending an email to clinical@polyganics.com. Any questions in advance can also be submitted using this e-mail adress.
- Yes, I will attend the 1st SHIELDS Investigator Meeting on March-3, 2021; 6-8PM
- No, I cannot attend the 1st SHIELDS Investigator Meeting on March-3, 2021; 6-8PM
A summary and/or recording of the IM meeting will be made available afterwards to all study participants.
We look forward to discussing the study progress and cases with all of you!
Announcement partnership E-AHPBA
We are proud to announce that the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) and Polyganics agreed to enter into a Strategic Partnership. The recent health crisis has reaffirmed the need for close cooperation between medical associations and industry to ensure all can continue to support our members, customers, and affiliates effectively and efficiently. There are many synergies between the E-AHPBA and Polyganics, both currently have similar priorities to educate and promote innovation. The arrangement is one whereby the E-AHPBA and Polyganics work closely to provide opportunities to surgeons, of all levels, across Europe, Africa and the Middle East. In 2021 we aim to get a broader perspective on the standard of care in pancreas and liver surgery; a pivotal element for the randomized controlled study, SHIELDS-2, targeted to start by end of this year.

Reminder: Online instruction videos
To enable participants in the SHIELDS clinical study to use ACTISEAL® effectively, we have prepared online instructional videos.
Two separate videos are available for using the Sealing Device in Liver Surgery (below, left) and Pancreatic Surgery (below, right). In the Liver Surgery video, two different procedures are shown; one in which the device is applied on a relatively smooth resection surface, and one in which it is used in the remaining cavity after resection.