LIQOSEAL®, the first product in our Neurosurgery portfolio, is indicated for use as an adjunct to standard methods of cranial dural repair to provide watertight closure of the dura mater and reduce cerebrospinal fluid (CSF) leakage.
Welcome to the March issue of our quarterly LIQOSEAL® newsletter.
LIQOSEAL® was launched commercially in Europe in early 2020, after achieving CE-mark certification that January. Now, just over a year after launch, uptake of the device is accelerating and several centres are transitioning to use our innovative dura sealant patch as their standard of care for dural closure. The successful impact on the Neurosurgery market throughout Europe is a credit to you, our distribution partners.
As always with these newsletters, we aim to share the latest news on LIQOSEAL®. In this issue, look out for updates on:
- The commercial rollout of LIQOSEAL®
- Our new, smaller patch and extension of the LIQOSEAL® shelf life
- ENCASE II study preparations and progress
Thank you for taking the time to read this newsletter. If you have comments, questions, or we can help further in any way to support your business activities or events, please do not hesitate to get in touch.
LIQOSEAL® commercial roll out
We are pleased to report that the uptake of LIQOSEAL® throughout Europe continues to increase. We have received positive feedback from surgeons, in particular with regards to the performance and handling / ease of use of the device. Several centres are even now using LIQOSEAL® as their standard of care for dural closure, as a dedicated device for neurosurgical procedures.
Outside Europe there is also high interest in LIQOSEAL®. We are currently in the (registration) process of making the device available in other countries, including Jordan, Israel, Argentina and Australia in the near term. We are also preparing to register LIQOSEAL® in Brazil, South Korea, China and Japan.
As part of our commercial rollout, we hosted a successful Q&A session for distributors in February. During the event, Dr. Tristan van Doormaal (Neurosurgeon at the University Hospital Zurich (Switzerland) and University Medical Center (UMC) in Utrecht (The Netherlands) and Principal Investigator of ENCASE I) shared and reviewed a number of clinical case studies and answered related questions. To catch-up on the discussions from the Q&A, please watch:
Product update: 5×5 cm LIQOSEAL® now available and extended shelf-life
Recently, we have introduced an additional SKU for LIQOSEAL® – surgeons can now benefit from a new size of patch (5x5cm) as well as the original (8x8cm). We hope that the 5x5cm LIQOSEAL® offers a cost-effective solution for small incisions and closures that only need a minor adjunct. Having both products available in the OR provides improved flexibility, which we believe will increase use of our products.
Furthermore, we have been able to successfully extend the shelf life of our products, from 18 to 36 months after production. We trust that this will allow you more flexibility and further support your sales processes with hospitals.*
* Please note the storage and transport conditions remain unchanged. Presently we ship LIQOSEAL® within 72 hours in validated boxes using icepacks. Upon receipt at your office, please store them immediately in the freezer between -15°C and -30°C).
ENCASE II study
This month, we have taken another step towards entering the US market with LIQOSEAL®; we are happy to inform you that we received confirmation from the FDA that we can start ENCASE II in the USA. We are currently finalizing preparations to start enrolment for ENCASE II in April 2021.
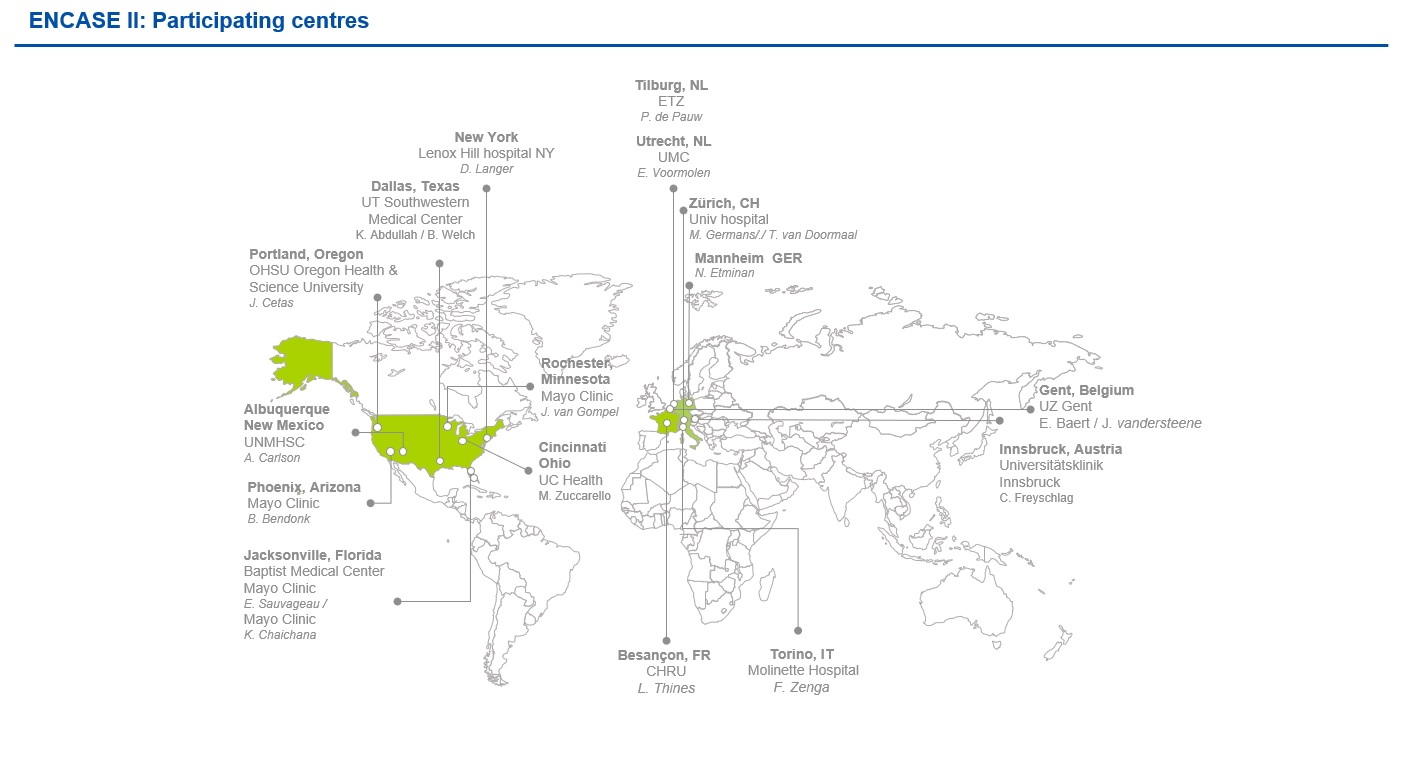
ENCASE II is a randomized, two-arm, multicenter study, evaluating the safety and efficacy of LIQOSEAL® in reducing CSF leakage following elective cranial surgery. The study will involve world-class clinical centers in Europe (8) and the US (10), with the option to extend this to include 20 sites in total worldwide. Figure 1 shows a map of these sites.
All of these centres expressed specific interest in participating in ENCASE II, recognizing the game-changing potential of LIQOSEAL® as a dedicated neurosurgical device. We are very happy to have these expert surgeons and their knowledgeable teams involved and we look forward to the start of the study.
Additional online material
At the end of 2020 we organized a webinar: ‘Reducing CSF leakage after Cranial Surgery’. To listen back to the content shared and contributions from all speakers, please click here.
You can also watch back presentations from the EANS VIRTUAL CONGRESS and the 32nd Annual Congress of the International Society for Medical Innovation and Technology (SMIT)