Welcome to the next issue of our LIQOSEAL – ENCASE II Clinical Study newsletter
With this newsletter, we aim to keep all participating clinical centers regularly updated with the latest information on the ENCASE II trial.
What you’ll find in this issue:
- First patients enrolled to ENCASE II in US and EU
- Overview of active clinical centres and number of patients enrolled
- 1-year ENCASE I data published in BMJ Open
- Pre-clinical LIQOSEAL data published in Journals of Materials Science: Materials in Medicine
- EANS booth, presentations and Investigator Meeting
Thank you for reading our ENCASE II newsletter. If you have comments, questions, or we can help further in any way to support enrolment for the study at your clinical centre, please get in touch.

Patient Enrollment has started: First patients treated in ENCASE II study in US and EU
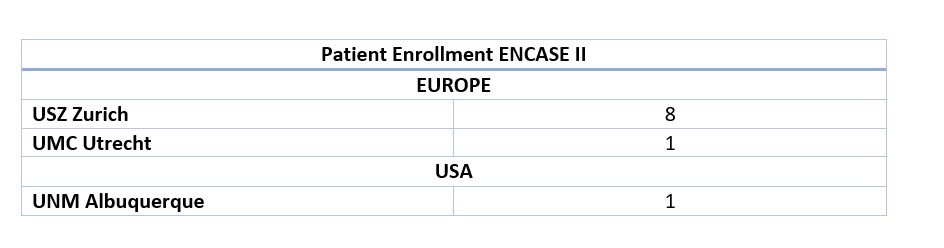
Following the first patient enrollments in Europe (in USZ-Zürich, UMC Utrecht), we are now proud to announce that the first patients have also been enrolled in the US.
We are delighted with the response and enthusiasm we have seen from participating surgeons. In the video you can hear first-hand from Dr. Andrew Carlson, Neurosurgeon at the University of New Mexico Hospitals in Albuquerque, New Mexico, and Global Coordinating Investigator for the study, why he chose to get involved in ENCASE II.
LIQOSEAL is a potentially game-changing device. This is a unique opportunity to make a meaningful impact for patients
– Dr. Andrew Carlson, Neurosurgeon at the University of New Mexico Hospitals in Albuquerque, and Global Coordinating Investigator for the ENCASE II study
ENCASE II – Patient Enrollment
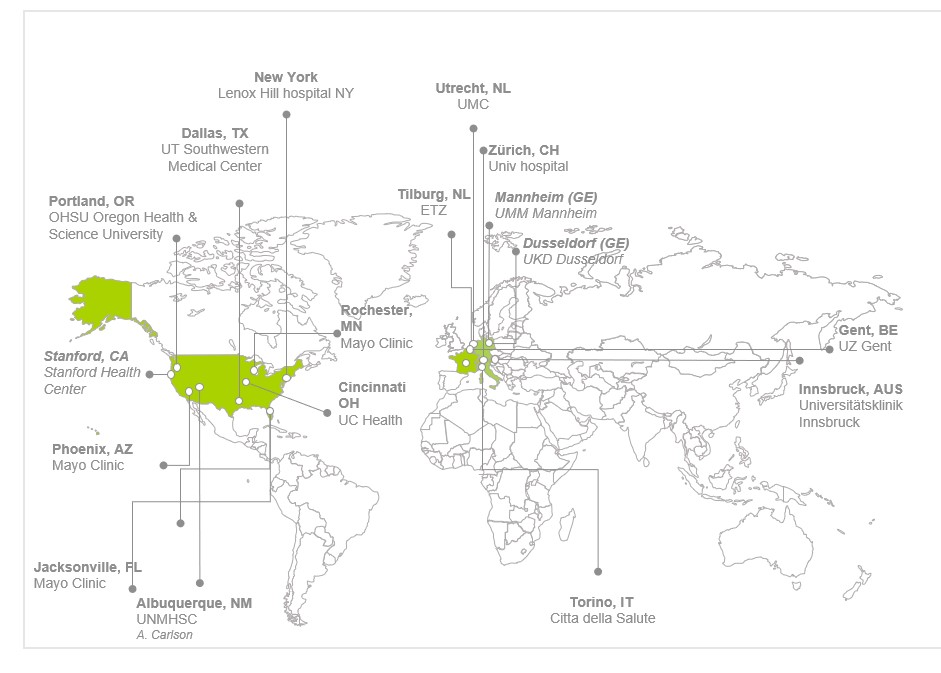
ENCASE II – Participating Centres
1-year data ENCASE I published in BMJ Open
Polyganics achieved CE mark certification for LIQOSEAL in early 2020, based on positive 3-month data from ENCASE I. 12-month data have now been published in BMJ Open. The ENCASE I trial investigated the safety and performance of LIQOSEAL in reducing cerebrospinal fluid (CSF) leakage following elective cranial intradural surgery. The 12-month data, which was collected at sites in Switzerland and the Netherlands, showed no CSF leakage or clinically relevant swelling in any of the 40 patients enrolled, and no serious device-related adverse events were reported. We asked Dr Tristan van Doormaal, Neurosurgeon at the University Hospital Zurich and University Medical Center (UMC) in Utrecht, Principal Investigator of the study, and leader of the initial research project at the Brain Technology Institute (BTI) to comment on the data and publication:
CSF leakage is a well-known complication of neurosurgical procedures and a significant burden to patients and healthcare systems. LIQOSEAL has proven to significantly reduce CSF leakage compared to standard of care methods. I am pleased to have been involved in the ENCASE I trial and to have played my part in delivering this much-needed device to surgeons. Publication of the data in BMJ Open is recognition of the quality and impact of these results.
– Prof Dr Tristan van Doormaal, Neurosurgeon at the University Hospital Zurich and University Medical Center (UMC) in Utrecht, Principal Investigator of the study, and leader of the initial research project at the Brain Technology Institute (BTI)
Pre-clinical LIQOSEAL data published in Journals of Materials Science: Materials in Medicine
We are happy to announce that the pre-clinical ex-vivo data on LIQOSEAL have been published in the Journal of Materials Science: Materials in Medicine (JMSM). Together with the publications of the ENCASE I protocol and the ENCASE I results, this helps to further strengthen the clinical evidence for LIQOSEAL.
The publication “Ex vivo Evaluation of a Multi-layered Sealant Patch for Watertight Dural Closure: Cranial and Spinal Models” can be found here.*
Liqoseal is capable of achieving a strong watertight seal of a dural defect in conditions simulating cranial and spinal application on the dura. Liqoseal was shown to have a high probability of preventing CSF leakage compared with current clinically used sealants.
Our presence at conferences and upcoming investigator meetings
Unfortunately the EANS Meeting in Hamburg has been changed to a virtual meeting and our activities will be rescheduled. We are now planning the Online Investigator Meeting ENCASE-II on Thursday October 7th 18.00 CET. Please save the date – additional information will be shared shortly
* Please note – LIQOSEAL is not intended for use in the spine: LIQOSEAL is indicated for use as an adjunct to standard methods of cranial dural repair to provide a watertight closure of the dura mater and reduce cerebrospinal fluid (CSF) leakage. For more information, please read the Instructions for Use




