Welcome to the first issue of our LIQOSEAL® – ENCASE II Clinical Study newsletter.
LIQOSEAL® is the first product in our Neurosurgery portfolio. The easy-to-use dural sealant patch is indicated for use as an adjunct to standard methods of cranial dural repair, to provide watertight closure of the dura mater and reduce cerebrospinal fluid leakage.
Based on the results of our ENCASE I clinical study, LIQOSEAL® achieved CE-mark certification in January 2020. Now, just over a year after launch, LIQOSEAL® is commercially available throughout Europe via a network of renowned distributors, and uptake of the device is accelerating, with several centres now using our patch as their standard of care for dural closure.
Outside Europe, we are in the (registration) process of making LIQOSEAL® available in other countries, including Australia, Israel and Argentina in the near term.
We recently added a new size of patch (5x5cm) as well to the current patch (8x8cm). Surgeons can use this 5x5cm patch for smaller incisions and closures that only need a minor adjunct.
The next step for LIQOSEAL® is the ENCASE II study, which will enable us to submit the device to the FDA for Premarket Approval. ENCASE II is a randomized, two-arm, multicenter study, evaluating the safety and efficacy of LIQOSEAL® in reducing CSF leakage following elective cranial surgery.
With this newsletter, we aim to keep all participating clinical centers regularly updated with the latest information on this trial.
We are happy to inform you that we received confirmation from FDA that we can start ENCASE II in the USA.
In this issue, look out for:
- The clinical sites involved & next steps for participating centres
- Notice of upcoming Investigator Meeting
- Access to online training
Thank you for reading this first issue of our ENCASE II newsletter. If you have comments, questions, or we can help further in any way to support enrolment for the study at your clinical centre, please get in touch.
Clinical sites involved & next steps for participating centres
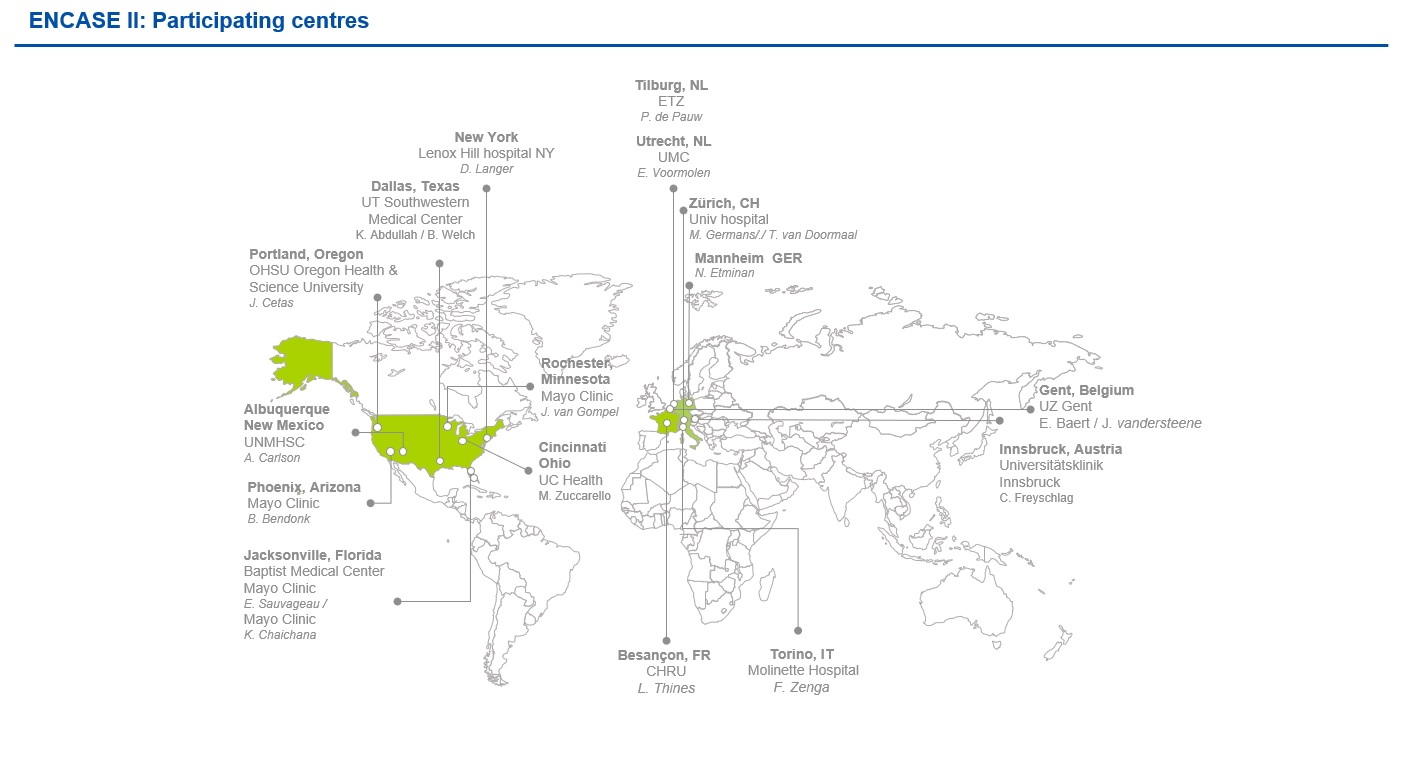
As a multicenter, prospective study, ENCASE II will involve 8 European sites and 10 US sites, with the option to extend this to include 20 sites in total. We are delighted to involve these world-class centres in our study, with their knowledgeable and experienced teams. Figure 1 shows a map of these sites.
During March and April, you will be contacted by our CRO, IQVIA MedTech (previously known as Genae), and/or Polyganics’ clinical team to finalize submission packages and schedule a site initiation visit (SIV). The SIV will be conducted by Polyganics in collaboration with IQVIA MedTech.
Save the date – Investigator Meeting 10 May 2021 at 6-7 PM CET
We are preparing for the first online ENCASE II Investigator Meeting on 10 May and would cordially invite you to participate participate. Watch out for your personal invite shortly.
At the Investigator meeting, we will share and discuss up-to-date experiences, results and practical tips for performance, as well as any relevant updates to the study procedures. Andrew Carlson, MD (MUNMSC Albuquerque) and Tristan van Doormaal, MD, PhD (UMC Utrecht-NL and USZ Zurich-CH), Principal Investigators of the ENCASE II study will present some cases.
The meeting agenda of this first IM is as follows:
- Encase I results
- Protocol: short recap
- Application of LIQOSEAL® during ENCASE II
- Status trial (enrolment and planning)
- Q&A
We look forward to discussing the study progress and cases with all of you then! A summary and/or recording of the meeting will be made available afterwards to all study participants.
Online training program
We have developed online training to support the ENCASE II study, to enable you to familiarize yourselves with the study and how to apply the LIQOSEAL® device ahead of the site initiation visit (SIV).
You will receive a personal passcode shortly, in order to access the training as well as additional background information on ENCASE II at your convenience at: www.encase-dura.com




